A) formation of a covalent bond
B) formation of an ionic bond
C) formation of a hydrogen bond
D) formation of an anion with a net charge of +1 and a cation with a net charge of -1
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of sulfur is 16,which indicates that a sulfur atom contains
A) 16 neutrons.
B) 16 protons.
C) 16 protons and 16 neutrons.
D) 8 electrons in its outermost electron shell.
E) 8 protons and 8 neutrons.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In ammonium chloride salt (NH4Cl) ,the anion is a single chloride ion, (Cl¯) .What is the cation of NH4Cl salt?
A) N,with a charge of +1
B) NH,with a charge of +1
C) H3,with a charge of +1
D) NH4,with a charge of +1
E) NH4,with a charge of +4
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 10-3 M NaOH solution?
A) pH 3
B) pH 8
C) pH 9
D) pH 10
E) pH 11
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Molybdenum has an atomic number of 42.Several common isotopes exist,with mass numbers of 92,94,95,96,97,98,and 100.Therefore,which of the following is true?
A) The isotopes of molybdenum can have between 50 and 58 neutrons.
B) The isotopes of molybdenum have different numbers of valence electrons.
C) The isotopes of molybdenum can have between 50 and 58 protons.
D) The isotopes of molybdenum can have between 92 and 100 electrons.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider two solutions: solution X has a pH of 4;solution Y has a pH of 7.From this information,we can reasonably conclude that
A) solution Y has no free hydrogen ions (H+) because the solution is neutral.
B) the concentration of hydrogen ions in solution X is 30 times greater than the concentration of hydrogen ions in solution Y.
C) the concentration of hydrogen ions in solution Y is 1,000 times greater than the concentration of hydrogen ions in solution X.
D) the concentration of hydrogen ions in solution X is 3 times greater than the concentration of hydrogen ions in solution Y.
E) the concentration of hydrogen ions in solution X is 1,000 times greater than the concentration of hydrogen ions in solution Y.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
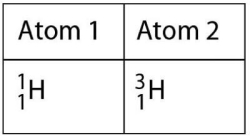 Figure 2.1
Which of the following best describes the relationship between the atoms described in Figure 2.1?
Figure 2.1
Which of the following best describes the relationship between the atoms described in Figure 2.1?
A) They are compounds.
B) They are polymers.
C) They are isotopes.
D) They contain 1 and 3 protons,respectively.
E) They each contain 1 neutron.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that acid precipitation can damage marine corals by
A) buffering ocean waters.
B) decreasing the H+ concentration in oceans.
C) increasing the OH- concentration in oceans.
D) decreasing the concentration of carbonate ions in oceans.
E) decreasing the calcium ion concentration in oceans.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hydroxyl ion (OH-) concentration of a solution of pH 8?
A) 8 M
B) 8 × 10-6 M
C) 0.01 M
D) 10-8 M
E) 10-6 M
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon dioxide (CO2) is readily soluble in water,according to the equation CO2 + H2O ↔ H2CO3.Carbonic acid (H2CO3) is a weak acid.If CO2 is bubbled into a beaker containing pure,freshly distilled water,which of the following graphs correctly describes the results?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An equal volume (5 mL) of milk of magnesia from a freshly opened bottle is added to each of the following solutions.After complete mixing,which of the mixtures will have the lowest pH?
A) 100 mL of pure water
B) 100 mL of freshly brewed coffee
C) 100 mL of household cleanser containing 0.5 M ammonia
D) 100 mL of freshly squeezed lemon juice
E) 100 mL of household cleanser containing 0.5 M bleach
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Buffers are substances that help resist shifts in pH by
A) releasing H+ to a solution when acids are added.
B) accepting OH- from a solution when bases are added.
C) releasing OH- to a solution when bases are added.
D) accepting H+ from a solution when acids are added.
E) accepting OH- from a solution when acids are added.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Unequal sharing of electrons between atoms will result in which of the following interactions?
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrophobic interaction
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The chemical behavior of an atom depends primarily upon which of the following?
A) the number of neutrons in the nucleus
B) the number of protons in the nucleus
C) the number of electrons in the valence shell
D) the total number of electrons contained by the atom
E) the number of electron shells contained by the atom
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar mass of glucose (C6H12O6) is 180 g/mol.Which of the following procedures should you carry out to make a 0.5 M solution of glucose?
A) Dissolve 0.5 g of glucose in a small volume of water,and then add more water until the total volume of the solution is 1 L.
B) Dissolve 90 g of glucose in a small volume of water,and then add more water until the total volume of the solution is 1 L.
C) Dissolve 180 g of glucose in a small volume of water,and then add more water until the total volume of the solution is 1 L.
D) Dissolve 0.5 g of glucose in 1 L of water.
E) Dissolve 180 g of glucose in 0.5 L of water.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 121 - 135 of 135
Related Exams